 金牌会员
金牌会员
 已认证
已认证
2.9.19. PARTICULATE CONTAMINATION: SUB-VISIBLE PARTICLES
2.9.19. 颗粒物污染:亚可见颗粒物
Particulate contamination of injections and infusions consists of extraneous, mobile undissolved particles, other than gas bubbles, unintentionally present in the solutions.
注射剂和输液的微粒污染包括在溶液中无意中存在的外来的、可移动的未溶解颗粒,而不是气泡。
For the determination of particulate contamination 2 procedures, Method 1 (Light Obscuration Particle Count Test) and Method 2 (Microscopic Particle Count Test), are specified hereinafter. When examining injections and infusions for sub-visible particles, Method 1 is preferably applied. However, it may be necessary to test some preparations by the light obscuration particle count test followed by the microscopic particle count test to reach a conclusion on conformance to the requirements.
对于颗粒物污染的测定包含两种方法,下文进行了详细介绍:方法1(光阻法颗粒计数试验)和方法2(显微颗粒物计数试验)。当检查注射剂和输液中是否存在亚可见颗粒时,最好使用方法1。然而,可能有必要通过光阻法颗粒计数试验和显微颗粒计数试验来测试某些制剂,以得出符合要求的结论。
Not all parenteral preparations can be examined for sub-visible particles by one or both of these methods. When Method 1 is not applicable, e.g. in case of preparations having reduced clarity or increased viscosity, the test is carried out according to Method 2. Emulsions, colloids, and liposomal preparations are examples. Similarly, products that produce air or gas bubbles when drawn into the sensor may also require microscopic particle count testing. If the viscosity of the preparation to be tested is sufficiently high so as to preclude its examination by either test method, a quantitative dilution with an appropriate diluent may be made to decrease viscosity, as necessary, to allow the analysis to be performed.
并非所有非肠道制剂都可以通过这些方法中的一种或两种方法检查亚可见颗粒。例如在制剂的透明度降低或粘度增加的情况下,方法 1 不适用时,应根据方法2进行测试,如乳液、胶体和脂质体制剂等。 同样,在吸入传感器时会产生空气或气泡的产品也可能需要进行显微颗粒物计数测试。 如果待测制剂的粘度足够高以至于无法通过任一测试方法对其进行检查,则可以根据需要,用适当的稀释剂进行定量稀释以降低粘度,以便进行分析。
The results obtained in examining a discrete unit or group of units for particulate contamination cannot be extrapolated with certainty to other units that remain untested. Thus, statistically sound sampling plans must be developed if valid inferences are to be drawn from observed data to characterize the level of particulate contamination in a large group of units.
在检查一个离散单元或一组单元的微粒污染时获得的结果不能肯定地外推到其他未测试的单元。 因此,如果要从观察到的数据中得出有效的推论,以表征一大组单位中的颗粒污染水平,就必须制定统计上合理的抽样计划。
METHOD 1. LIGHT OBSCURATION PARTICLE COUNTTEST
光阻法颗粒计数实验
Use a suitable apparatus based on the principle of light blockage which allows an automatic determination of the size of particles and the number of particles according to size. The apparatus is calibrated using suitable certified reference materials consisting of dispersions of spherical particles of known sizes between 10 µm and 25 µm. These standard particles are dispersed in particle-free water R. Care must be taken to avoid aggregation of particles during dispersion.
根据光阻原理,使用合适的仪器,可以根据大小自动确定颗粒大小和颗粒数量。使用合适的认证标准物质对仪器进行校准,该标准物质由已知尺寸在10 µm到25 µm之间的球形颗粒的分散体组成。这些标准颗粒分散在无颗粒水中。分散过程中必须注意避免颗粒聚集。
General precautions 一般注意事项
The test is carried out under conditions limiting particulate contamination, preferably in a laminar-flow cabinet. Very carefully wash the glassware and filtration equipment used, except for the membrane filters, with a warm detergent solution and rinse with abundant amounts of water to remove all traces of detergent. Immediately before use, rinse the equipment from top to bottom, outside and then inside, with particle-free water R.
实验在限制颗粒污染的条件下进行,最好在层流柜中进行。使用温热的清洁剂溶液仔细清洗所用的玻璃器皿和过滤设备(膜式过滤器除外),并用大量水冲洗,以去除所有清洁剂痕迹。使用前,用无颗粒水从上到下、从外到内冲洗设备。
Take care not to introduce air bubbles into the preparation to be examined, especially when fractions of the preparation are being transferred to the container in which the determination is to be carried out.
注意不要将气泡引入待检查的制剂中,尤其是将制剂的部分转移到要进行测定的容器中时。
In order to check that the environment is suitable for the test, that the glassware is properly cleaned and that the water to be used is particle-free, the following test is carried out: determine the particulate contamination of 5 samples of particle-free water R, each of 5 mL, according to the method described below. If the number of particles of 10 µm or greater size exceeds 25 for the combined 25 mL, the precautions taken for the test are not sufficient. The preparatory steps must be repeated until the environment, glassware and water are suitable for the test。
为了检查环境是否适合试验,玻璃器皿是否正确清洁,所使用的水是否无颗粒,应进行以下试验:根据下述方法,测定5个无颗粒水样品的颗粒污染情况,每个样品5 mL。如果10 µm或更大粒径的颗粒数量超过25个,则为试验采取的预防措施是不够的。必须重复准备步骤,直到环境、玻璃器皿和水适合试验
Method方法
Mix the contents of the sample by slowly inverting the container 20 times successively. If necessary, cautiously remove the sealing closure. Clean the outer surfaces of the container opening using a jet of particle-free water R and remove the closure, avoiding any contamination of the contents. Eliminate gas bubbles by appropriate measures such as allowing to stand for 2 min or sonicating.
通过连续20次缓慢翻转容器,混合样品里的内容物。如有必要,小心地拆下密封盖。使用无颗粒水水流清洁容器开口的外表面,并拆下封盖,避免内容物受到任何污染。通过适当的措施消除气泡,例如静置2分钟或超声波处理。
For large-volume parenterals, single units are tested. For small-volume parenterals less than 25 mL in volume, the contents of 10 or more units are combined in a cleaned container to obtain a volume of not less than 25 mL; where justified and authorised, the test solution may be prepared by mixing the contents of a suitable number of vials and diluting to 25 mL with particle-free water R or with an appropriate solvent without contamination of particles when particle-free water R is not suitable. Small-volume parenterals having a volume of 25 mL or more may be tested individually.
对于大容量肠外注射剂,需要测试单个单元。对于体积小于25 mL的小容量肠外注射剂,将10个或更多个的内容物组合在一个清洁的容器中,以获得不小于25 mL的体积的溶液;在合理和授权的情况下,可通过混合适当数量的小瓶中的内容物,并用无颗粒水稀释至25 mL,或在无颗粒水不适用时,用无颗粒污染的适当溶剂稀释至25 mL来制备试验溶液。体积为25 mL或以上的小容量非肠道药物试剂可单独进行试验。
Powders for parenteral administration are reconstituted with particle-free water R or with an appropriate solvent without contamination of particles when particle-free water R is not suitable.
用于肠外给药的粉末用无颗粒水重新配制,或当无颗粒水不适用时,使用适当的无颗粒污染的溶剂重新进行配制。
The number of test specimens must be adequate to provide a statistically sound assessment. For large-volume parenterals or for small-volume parenterals having a volume of 25 mL or more, fewer than 10 units may be tested, based on an appropriate sampling plan。
测试样本的数量必须足够,以提供统计上合理的评估。根据适当的抽样计划,对于大容量或25 mL以上的小容量肠外静脉,可检测小于10个样品
Remove 4 portions, each of not less than 5 mL, and count the number of particles equal to or greater than 10 µm and 25 µm. Disregard the result obtained for the first portion, and calculate the mean number of particles for the preparation to be examined.
取下4份样品,每份不少于5 mL,并计算等于或大于10 µm和25 µm的颗粒数。忽略第一份的结果,并计算待检查制剂的平均颗粒数。
Evaluation评估
For preparations supplied in containers with a nominal volume of more than 100 mL, apply the criteria of test 1.A.
For preparations supplied in containers with a nominal volume of less than 100 mL, apply the criteria of test 1.B.
♦For preparations supplied in containers with a nominal volume of 100 mL, apply the criteria of test 1.B. ♦
If the average number of particles exceeds the limits, test the preparation by the microscopic particle count test.
对于在标称体积超过100 mL的容器中提供的制剂,应采用试验1A的标准。
对于在标称体积小于100 mL的容器中提供的制剂,采用试验1B的标准。
对于在标称体积为100 mL的容器中提供的制剂,采用试验1B的标准。
如果平均颗粒数超过限值,则通过显微颗粒计数测试对制剂进行测试。
Test 1.A – Solutions for infusion or solutions for injection supplied in containers with a nominal content of more than 100 mL
The preparation complies with the test if the average number of particles present in the units tested does not exceed 25 per millilitre equal to or greater than 10 µm and does not exceed 3 per millilitre equal to or greater than 25 µm.
试验1A–输液溶液或注射溶液,装在标称含量超过100 mL的容器中。
如果被测单位样品中粒径等于或大于10 µm的颗粒的平均数量不超过每毫升25个,且粒径等于或大于25 µm的颗粒平均数量不超过每毫升3个,则制剂符合试验要求。
Test 1.B – Solutions for infusion or solutions for injection supplied in containers with a nominal content of less than 100 mL
The preparation complies with the test if the average number of particles present in the units tested does not exceed 6000 per container equal to or greater than 10 µm and does not exceed 600 per container equal to or greater than 25 µm.
试验1B–输液溶液或注射溶液,装在标称含量小于100 mL的容器中。
如果被测单元中粒径等于或大于10 µm的颗粒的平均数量不超过6000个/容器,且粒径等于或大于25 µm的颗粒平均数量不超过600个/容器,则制剂符合试验要求。
METHOD 2. MICROSCOPIC PARTICLE COUNT TEST
显微颗粒计数
Use a suitable binocular microscope, filter assembly for retaining particulate contamination and membrane filter for examination.
使用合适的双目显微镜、过滤器组件(用于保留颗粒污染物)和薄膜过滤器(用于检查)。
The microscope is equipped with an ocular micrometer calibrated with an objective micrometer, a mechanical stage capable of holding and traversing the entire filtration area of the membrane filter, 2 suitable illuminators to provide episcopic illumination in addition to oblique illumination, and is adjusted to 100 ± 10 magnifications.
该显微镜配有一个目镜测微计(使用物镜测微计校准)、一个机械工作台(能够容纳并穿过膜过滤器的整个过滤区域)、2个合适的照明器(除了倾斜照明外,还提供反射照明),并将其调整为100±10倍。
The ocular micrometer is a circular diameter graticule (see Figure 2.9.19.-1.) and consists of a large circle divided by crosshairs into quadrants, transparent and black reference circles 10 µm and 25 µm in diameter at 100 magnifications, and a linear scale graduated in 10 µm increments. It is calibrated using a stage micrometer that is certified by either a domestic or international standard institution. A relative error of the linear scale of the graticule within ± 2 per cent is acceptable. The large circle is designated the graticule field of view (GFOV).
目镜测微计是一个圆形直径分划器(见图2.9.19.-1)由一个被十字准线分成四个象限的大圆、100倍放大时直径为10 µm和25 µm的透明和黑色参考圆、以及以10 µm增量刻度的线性刻度组成。使用经过国内或国际标准机构认证的测微计对其进行校准。分划线性刻度的相对误差在±2%以内是可以接受的。大圆被指定为网格视场(GFOV)。
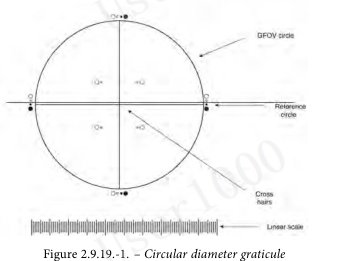
2 illuminators are required. One is an episcopic brightfield illuminator internal to the microscope, the other is an external, focusable auxiliary illuminator adjustable to give reflected oblique illumination at an angle of 10-20°.
需要2个照明器。一个是显微镜内部的一个反射式亮场照明器,另一个是一个可调的外部可聚焦辅助照明器,以提供10-20°角的反射斜照明。
The filter assembly for retaining particulate contamination consists of a filter holder made of glass or other suitable material, and is equipped with a vacuum source and a suitable membrane filter.
用于保留颗粒污染物的过滤器组件包括由玻璃或其他合适材料制成的过滤器支架,并配备有真空源和合适的膜过滤器。
The membrane filter is of suitable size, black or dark grey in colour, non-gridded or gridded, and 1.0 µm or finer in nominal pore size.
膜过滤器尺寸合适,颜色为黑色或深灰色,无网格或网格,标称孔径为1.0 µm或更小。
General precautions 一般注意事项
The test is carried out under conditions limiting particulate contamination, preferably in a laminar-flow cabinet.
Very carefully wash the glassware and filter assembly used, except for the membrane filter, with a warm detergent solution and rinse with abundant amounts of water to remove all traces of detergent. Immediately before use, rinse both sides of the membrane filter and the equipment from top to bottom, outside and then inside, with particle-free water R.
试验在限制颗粒污染的条件下进行,最好在层流柜中进行。
使用温热的清洁剂溶液仔细清洗所用的玻璃器皿和过滤器组件(膜式过滤器除外),并用大量水冲洗,以去除所有清洁剂痕迹。使用前,用无颗粒水从上到下、从外到内冲洗膜过滤器和设备的两侧。
In order to check that the environment is suitable for the test, that the glassware and the membrane filter are properly cleaned and that the water to be used is particle-free, the following test is carried out: determine the particulate contamination of a 50 mL volume of particle-free water R according to the method described below. If more than 20 particles 10 µm or larger in size or if more than 5 particles 25 µm or larger in size are present within the filtration area, the precautions taken for the test are not sufficient. The preparatory steps must be repeated until the environment, glassware, membrane filter and water are suitable for the test.
为了检查环境是否适合试验,玻璃器皿和膜过滤器是否正确清洁,所使用的水是否无颗粒,应进行以下试验:根据下述方法测定50 mL无颗粒水的颗粒污染情况。如果过滤区域内存在20个以上粒径为10 µm或更大的颗粒,或5个以上粒径为25 µm或更大的颗粒,则试验所采取的预防措施是不够的。必须重复准备步骤,直到环境、玻璃器皿、膜过滤器和水适合试验。
Method方法
Mix the contents of the samples by slowly inverting the container 20 times successively. If necessary, cautiously remove the sealing closure. Clean the outer surfaces of the container opening using a jet of particle-free water R and remove the closure, avoiding any contamination of the contents.
通过将容器连续缓慢翻转20次来混合样品内容物。如有必要,小心地拆下密封盖。使用无颗粒水水流清洁容器开口的外表面,并拆下封盖,避免内容物的任何污染。
For large-volume parenterals, single units are tested. For small-volume parenterals less than 25 mL in volume, the contents of 10 or more units are combined in a cleaned container; where justified and authorised, the test solution may be prepared by mixing the contents of a suitable number of vials and diluting to 25 mL with particle-free water R or with an appropriate solvent without contamination of particles when particle-free water R is not suitable. Small-volume parenterals having a volume of 25 mL or more may be tested individually.
对于大容量肠外注射,需要测试单个样品。对于体积小于25 mL的小容量肠外注射剂,将10个或更多个的样品组合在一个清洁的容器中;在合理和授权的情况下,可通过混合适当数量的小瓶中的内容物,并用无颗粒水稀释至25 mL,当无颗粒水不适用时,可用无颗粒污染的适当溶剂稀释至25 mL来制备试验溶液。体积为25 mL或以上的小容量非肠道药物可单独进行试验。
Powders for parenteral administration are constituted with particle-free water R or with an appropriate solvent without contamination of particles when particle-free water R is not suitable.
用于肠外给药的粉末由无颗粒水配置,或当无颗粒水不适用时,使用不含污染颗粒的溶剂重新配制。
The number of test specimens must be adequate to provide a statistically sound assessment. For large-volume parenterals or for small-volume parenterals having a volume of 25 mL or more, fewer than 10 units may be tested, based on an appropriate sampling plan.
试样数量必须足以提供统计上合理的评估。对于大容量肠外注射剂或体积大于等于25 mL的小容量肠外注射剂,根据适当的取样计划,可测试少于10个样品。
Wet the inside of the filter holder fitted with the membrane filter with several millilitres of particle-free water R. Transfer to the filtration funnel the total volume of a solution pool or of a single unit, and apply vacuum. If needed, add stepwise a portion of the solution until the entire volume is filtered. After the last addition of solution, begin rinsing the inner walls of the filter holder by using a jet of particle-free water R. Maintain the vacuum until the surface of the membrane filter is free from liquid. Place the filter in a Petri dish and allow the filter to air-dry with the cover slightly ajar. After the filter has been dried, place the Petri dish on the stage of the microscope, scan the entire membrane filter under the reflected light from the illuminating device, and count the number of particles that are equal to or greater than 10 µm and the number of particles that are equal to or greater than 25 µm. Alternatively, partial filter count and determination of the total filter count by calculation is allowed. Calculate the mean number of particles for the preparation to be examined.
用几毫升无颗粒水湿润装有薄膜过滤器的过滤器支架内部。将溶液或单个样品转移到过滤漏斗中,并施加真空。如果需要,逐步添加一部分溶液,直到整个体积被过滤。最后一次添加溶液后,使用无颗粒水水流开始冲洗过滤器支架的内壁。保持真空,直到过滤膜表面没有液体。将过滤膜放置在有盖培养皿中,让过滤膜风干,盖子稍微半开。过滤膜干燥后,将皮氏培养皿放在显微镜台上,在照明设备的反射光下扫描整个膜过滤器,并计算等于或大于10 µm的颗粒数量和等于或大于25 µm的颗粒数量。或者,如果计算方式允许,可以对部分滤膜上的颗粒计数,并决定总滤膜上的颗粒数量。计算待检查制剂的平均颗粒数。
The particle sizing process with the use of the circular diameter graticule is carried out by transforming mentally the image of each particle into a circle and then comparing it to the 10 µm and 25 µm graticule reference circles. Thereby the particles are not moved from their initial locations within the graticule field of view and are not superimposed on the reference circles for comparison. The inner diameter of the transparent graticule reference circles is used to size white and transparent particles, while dark particles are sized by using the outer diameter of the black opaque graticule reference circles.
通过将每个颗粒的图像转换成一个圆,然后将其与10 µm和25 µm的分划参考圆进行比较,使用圆形直径分划的颗粒尺寸来执行颗粒尺寸测定过程。因此,粒子不会从网格视场内的初始位置移动,也不会叠加在参考圆上进行比较。透明分划参考圆的内径用于确定白色和透明粒子的大小,而黑色粒子的大小则使用黑色不透明分划参考圆的外径进行确定。
In performing the microscopic particle count test do not attempt to size or enumerate amorphous, semi-liquid, or otherwise morphologically indistinct materials that have the appearance of a stain or discoloration on the membrane filter. These materials show little or no surface relief and present a gelatinous or film-like appearance. In such cases the interpretation of enumeration may be aided by testing a sample of the solution by the light obscuration particle count test.
在进行微观颗粒计数试验时,不要试图测量或列举无定形、半液体或其他形态不明显的材料,这些材料在膜过滤器上出现污渍或变色。这些材料很少或没有表面浮雕,呈现胶状或薄膜状外观。在这种情况下,可通过光阻法粒子计数测试溶液样品来辅助解释计数。
Evaluation评估
For preparations supplied in containers with a nominal volume of more than 100 mL, apply the criteria of test 2.A.
For preparations supplied in containers with a nominal volume of less than 100 mL, apply the criteria of test 2.B.
♦For preparations supplied in containers with a nominal volume of 100 mL, apply the criteria of test 2B.♦
If the average number of particles exceeds the limits, test the preparation by the microscopic particle count test.
对于在标称体积大于100 mL的容器中提供的制剂,应采用试验2 A.的标准。
对于在标称体积小于100 mL的容器中提供的制剂,采用试验2 B的标准。
♦对于在标称体积为100 mL的容器中提供的制剂,采用试验2 B的标准。♦
如果平均颗粒数超过限值,则通过显微颗粒计数测试对制剂进行测试。
Test 2.A – Solutions for infusion or solutions for injection supplied in containers with a nominal content of more than 100 mL.
The preparation complies with the test if the average number of particles present in the units tested does not exceed 12 per millilitre equal to or greater than 10 µm and does not exceed 2 per millilitre equal to or greater than 25 µm.
试验2A–输液溶液或注射溶液,装在标称含量超过100 mL的容器中。
如果被测单位中颗粒尺寸等于或大于10 µm的平均数量不超过每毫升12个,且颗粒尺寸等于或大于25 µm的平均数量不超过每毫升2个,则制剂符合试验要求。
Test 2.B – Solutions for infusion or solutions for injection supplied in containers with a nominal content of less than 100 mL
The preparation complies with the test if the average number of particles present in the units tested does not exceed 3000 per container equal to or greater than 10 µm and does not exceed 300 per container equal to or greater than 25 µm.
试验2B–在标称含量小于100 mL的容器中提供的输液溶液或注射溶液。
如果被测单元中存在的尺寸等于或大于10 µm的颗粒平均数不超过3000个/容器,且尺寸大于等于25 um的颗粒平均数不超过300个/容器,则制剂符合试验要求。
检测结果判定标准总结:
方法 | 光阻法 | 显微计数法 | ||
粒径 | ≥10 μm | ≥25 μm | ≥10 μm | ≥ 25 μm |
体积≤100 mL | 6000/容器 | 600/容器 | 3000/容器 | 300/容器 |
体积>100 mL | <25/mL | <3/mL | <12/mL | <2/mL |



